How Do You Calculate The Concentration Of Naoh In A Titration . \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. \ce{naoh}\) is required to reach the end. Web calculating ph for titration solutions: Web suppose that a titration is performed and \(20.70 \: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web calculate the molarity of the sulfuric acid. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium.
from www.chegg.com
M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Web suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web calculating ph for titration solutions: Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. \ce{naoh}\) is required to reach the end. Web calculate the molarity of the sulfuric acid. Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved.
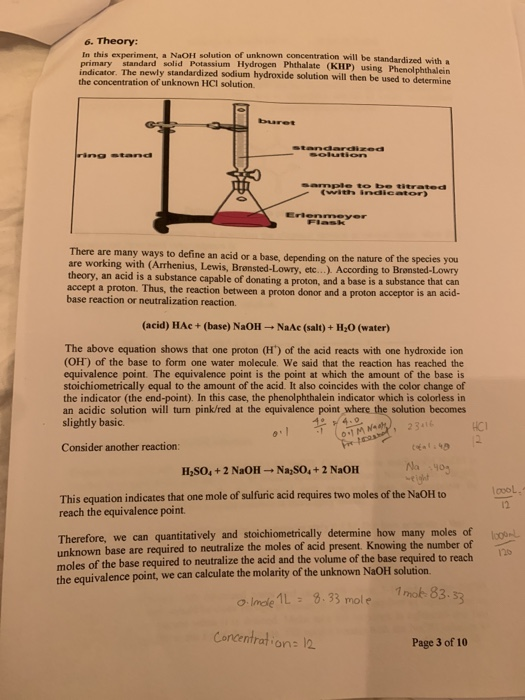
Solved 6. Theory In this experiment, a NaOH solution of
How Do You Calculate The Concentration Of Naoh In A Titration Web calculating ph for titration solutions: Web calculating ph for titration solutions: \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. Web suppose that a titration is performed and \(20.70 \: Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Web calculate the molarity of the sulfuric acid. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample.
From www.chegg.com
Solved CHEMISTRY STANDARDIZATION OF SODIUM HYDROXIDE How Do You Calculate The Concentration Of Naoh In A Titration Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. \ce{naoh}\) is required to reach the end.. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.vrogue.co
Solved Suppose The Concentration Of The Naoh Solution vrogue.co How Do You Calculate The Concentration Of Naoh In A Titration Web calculate the molarity of the sulfuric acid. \ce{naoh}\) is required to reach the end. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.vrogue.co
Solved Suppose The Concentration Of The Naoh Solution vrogue.co How Do You Calculate The Concentration Of Naoh In A Titration Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web suppose that a titration is performed and \(20.70. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH How Do You Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. Web calculate the molarity of the sulfuric acid. Web suppose that a titration is performed and \(20.70 \: Web. How Do You Calculate The Concentration Of Naoh In A Titration.
From fity.club
Titration Of Hcl With Naoh How Do You Calculate The Concentration Of Naoh In A Titration Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Web calculate the molarity of the sulfuric acid. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. Web titration is a method to determine the unknown concentration. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.chegg.com
Solved 6. Theory In this experiment, a NaOH solution of How Do You Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. Web suppose that a titration is performed and \(20.70 \: Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. Web to calculate concentration, we need to know the amount of naoh and the volume of. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.chegg.com
Solved Suppose the concentration of the NaOH solution was How Do You Calculate The Concentration Of Naoh In A Titration Web calculate the molarity of the sulfuric acid. Web suppose that a titration is performed and \(20.70 \: \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. \ce{naoh}\) is required to reach the end. Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Web. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding How Do You Calculate The Concentration Of Naoh In A Titration Web calculating ph for titration solutions: Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. Web suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.myxxgirl.com
Basics Of Titrations Part Iii Standardization Of Sodium Hydroxide My How Do You Calculate The Concentration Of Naoh In A Titration Web suppose that a titration is performed and \(20.70 \: Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. Web to calculate concentration,. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.chegg.com
Solved HCl(aq) + NaOH(aq)) NaCl(aq) + H2O(1) From the data How Do You Calculate The Concentration Of Naoh In A Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. Web calculate the molarity of the sulfuric acid. Web suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end.. How Do You Calculate The Concentration Of Naoh In A Titration.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts How Do You Calculate The Concentration Of Naoh In A Titration Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. Web calculating ph for titration solutions: \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web calculate the molarity of the sulfuric acid. Web to calculate concentration, we need. How Do You Calculate The Concentration Of Naoh In A Titration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How Do You Calculate The Concentration Of Naoh In A Titration Web suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow \ce{na_2so_4}. Web to calculate concentration, we need to know the amount of naoh. How Do You Calculate The Concentration Of Naoh In A Titration.
From shelbyscrosbyo.blob.core.windows.net
Titration Method Definition at shelbyscrosbyo blog How Do You Calculate The Concentration Of Naoh In A Titration Web calculate the molarity of the sulfuric acid. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. Web suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium.. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.nagwa.com
Question Video Calculating the Concentration of a Hydrochloric Acid How Do You Calculate The Concentration Of Naoh In A Titration Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. Web calculate the molarity of the sulfuric acid. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right). How Do You Calculate The Concentration Of Naoh In A Titration.
From annbjordanxo.blob.core.windows.net
Titration Chemistry Practice Problems How Do You Calculate The Concentration Of Naoh In A Titration Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. Web titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Web calculating ph for titration solutions: A. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co How Do You Calculate The Concentration Of Naoh In A Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. Web suppose that a titration is performed and \(20.70 \: Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Web in this part, you will determine the exact concentration of the ~0.1. How Do You Calculate The Concentration Of Naoh In A Titration.
From oneclass.com
OneClass please show all work Calculate the concentration of acid or How Do You Calculate The Concentration Of Naoh In A Titration Web calculate the molarity of the sulfuric acid. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Web to calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration.. How Do You Calculate The Concentration Of Naoh In A Titration.
From www.coursehero.com
[Solved] Determine the concentration of the NaOH titrant by using your How Do You Calculate The Concentration Of Naoh In A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. Web in this part, you will determine the exact concentration of the ~0.1 m sodium hydroxide solution using the potassium. \[\ce{h_2so_4} \left( aq \right) + 2 \ce{naoh} \left( aq \right) \rightarrow. How Do You Calculate The Concentration Of Naoh In A Titration.